Description
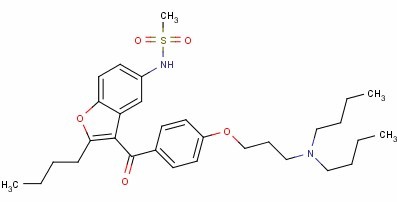
Product name: DronedaroneCAS: 141626-36-0
Molecular formula: C31H44N2O5S
Molecular weight: 556.764
Description: Dronedarone (development codename SR33589 and marketed as Multaq) is a drug by Sanofi-Aventis, mainly for the indication of cardiac arrhythmias. It was approved by the FDA on July 2, 2009. It was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion ( DCCV ) to maintain normal rhythm. However, the FDA did not approve dronedarone for reducing deaths. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in patients with moderate to severe CHF.
In order for dronedarone to be FDA approved the manufacturer had to add a Black box warning, stating that Multaq is contraindicated in patients with NYHA Class IV heart failure, or NYHA Class II¨CIII heart failure with a recent decompensation requiring hospitalization or referral to a specialized heart failure clinic." The FDA alerted healthcare professionals to rare cases of severe liver damage associated with the use of dronedarone.
More